The Gene Therapy Revolution
Gene therapy is redefining what’s possible in medicine. But its cost may define who it’s for.
The first weeks after a baby is born are supposed to be filled with sleepless wonders: the smell of newborn skin, tiny fingers wrapping around yours, and hopeful dreams about who this new person will become.
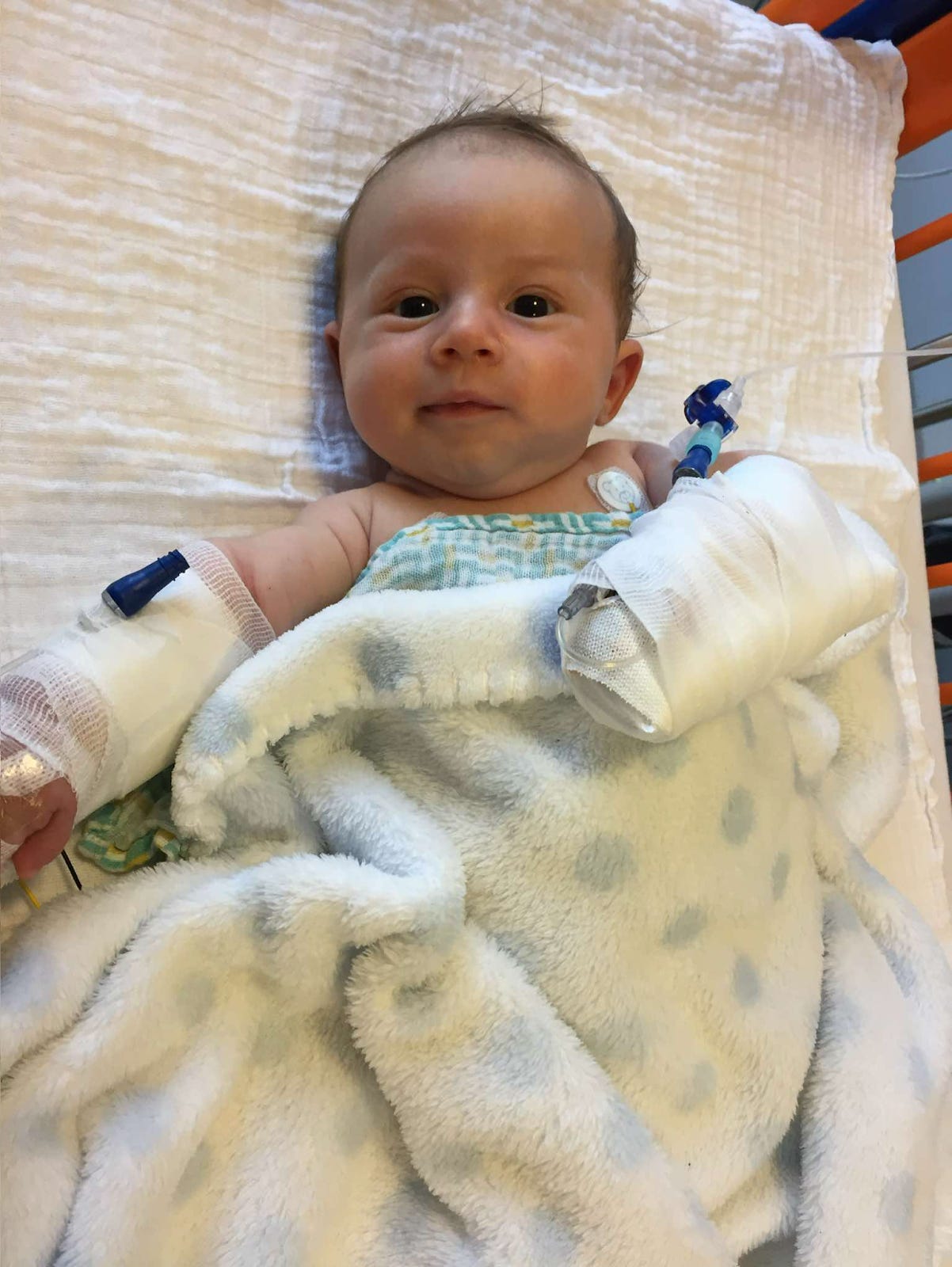
For Chiara and her family, those dreams were initially shattered by a call from the hospital four weeks after their son, Mattia, was born. On that call, Chiara learned that Mattia had a rare genetic disease called spinal muscular atrophy type 1 (SMA1). This diagnosis upended everything they imagined.
Overnight, Chiara and her family were thrust into a brand-new world. They now had to learn to care for their first child while navigating a life-threatening, rare disease.
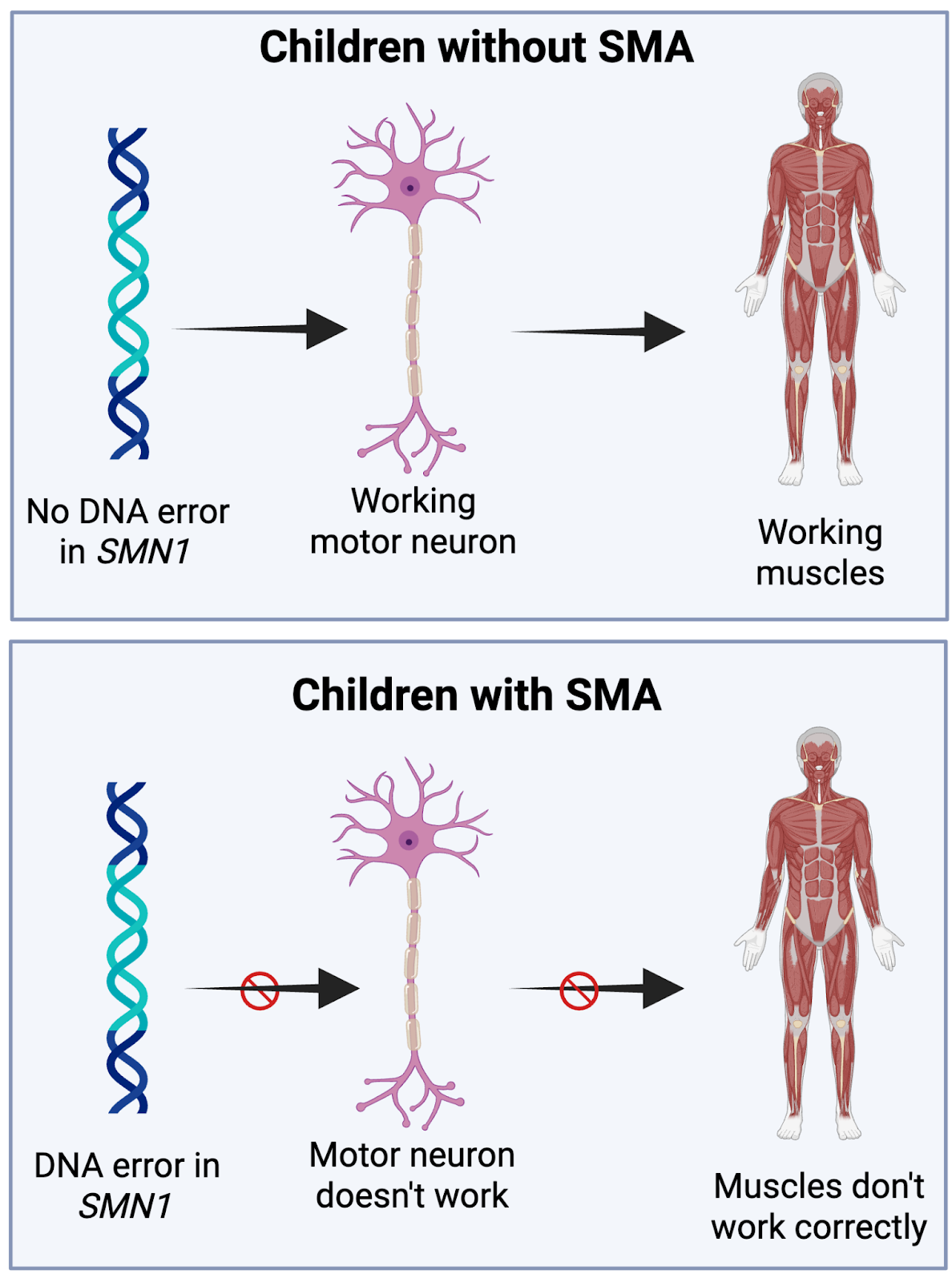
Because of an error in Mattia’s DNA, the nerve cells that control movement — known as motor neurons — did not function properly. Most kids with SMA1 live for less than two years because their bodies struggle to make their muscles move, including the ones they need to breathe.
Thankfully, a scientific advance changed everything.
At seven and a half weeks old, Mattia participated in a clinical trial and received a gene therapy treatment called Zolgensma.
What is gene therapy?
DNA is often called the “blueprint for life.” You can think of it as a recipe book filled with the instructions to make the proteins that build and run our bodies. When there is a mistake in one of those recipes — a mutation in a gene — the protein may not be made or may not function correctly. These mutations can cause genetic diseases like SMA1. In gene therapy, scientists treat the disease by editing the recipe or providing an alternative fix for what is missing.
While there is more than one type of gene therapy, Zolgensma uses a harmless virus to deliver the correct version of the gene to the motor neurons, allowing them to improve their function.
Mattia was not expected to live past two, but thanks to Zolgensma he is now six years old. He walks short distances with leg braces, laughs, plays, and attends school in a wheelchair.
The type of gene therapy given to Mattia and others can be difficult to manufacture, is extremely expensive, and doesn’t work for every type of genetic disease. Scientists continue to develop new methods to help more families like Mattia’s.
From bacteria to bedside
While Zolgensma showed what gene therapy can do, a newer tool promises to take these possibilities even further.
Many researchers are now using a groundbreaking technology called CRISPR (short for Clustered Regularly Interspaced Short Palindromic Repeats). CRISPR was first discovered as a defense mechanism that bacteria use to protect themselves against viruses. Today, it has become a powerful tool in medicine.
CRISPR acts as precise DNA scissors, allowing scientists to cut and edit DNA at almost any place in its strand. This makes CRISPR gene therapy potentially faster, more efficient, and more affordable than with other methods, and it’s already being used in clinical settings to change lives.
CRISPR and sickle cell disease
Sickle cell disease is an example of the success CRISPR is already having in the clinic. It’s caused by an error in the gene that makes hemoglobin, the protein in red blood cells that carries oxygen throughout the body. This small change alters the shape of red blood cells, making them stiff and crescent-shaped instead of flexible and round. As a result, these misshapen cells can clog blood vessels, leading to excruciating pain, organ damage, and even death.
In 2023, the FDA approved the first CRISPR-based gene therapy, called Casgevy, to treat patients with sickle cell disease.
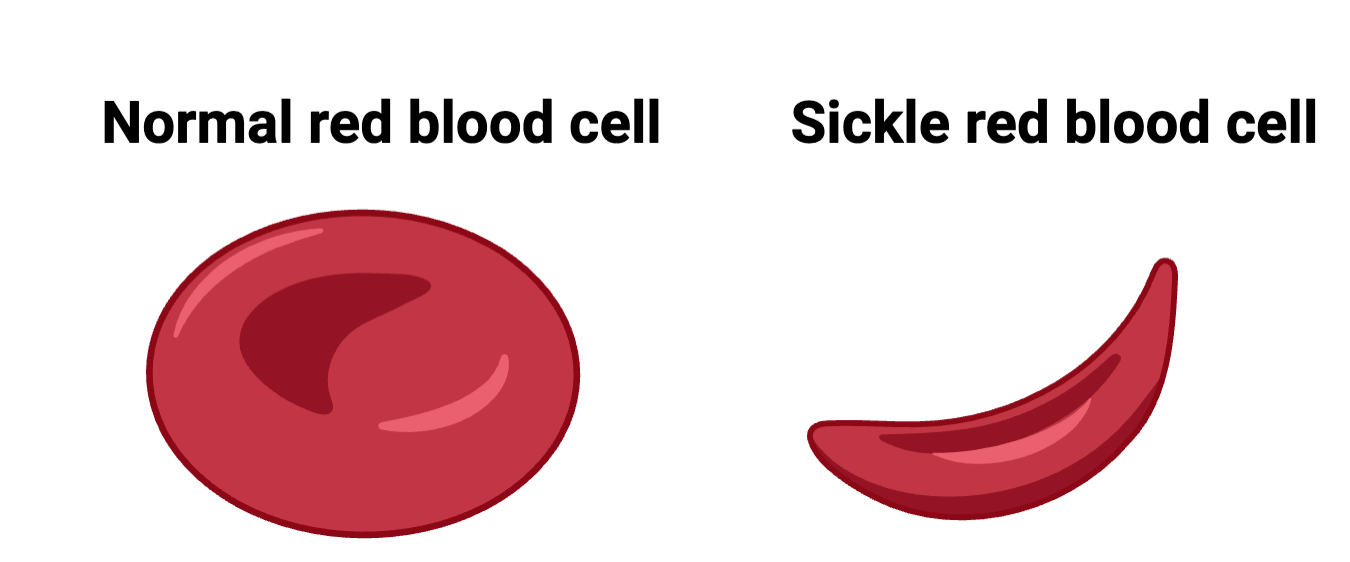
To understand how Casgevy works, let’s look more closely at hemoglobin. Before and shortly after birth, we all produce a fetal version of hemoglobin. A baby’s body then quickly switches over to making only the adult version. In people with sickle cell disease, it’s the adult form of hemoglobin that has the problem. Casgevy edits the patient’s own blood stem cells to restart production of fetal hemoglobin, keeping red blood cells flexible and healthy.
In clinical trials, patients who once needed frequent hospitalization for pain crises went more than two months without a single episode. For those living with sickle cell disease, this is life-changing.
CRISPR gives a baby new hope
The progress of gene therapies isn’t limited to sickle cell disease. In Spring 2025, scientists at the Children’s Hospital of Philadelphia, in collaboration with Penn Medicine, reported another major first. They designed a personalized CRISPR gene therapy to correct a mutation never seen before.
A baby, called KJ, was diagnosed with carbamoyl phosphate synthetase 1 (CPS1), a rare genetic disease in which the body cannot effectively remove ammonia from the blood. About half of infants with CPS1 die, and those who survive face many life-altering complications.
KJ’s team was able to design a custom CRISPR-based gene therapy to correct the specific error in his CPS1 gene. Reports indicate that he is now home and doing well. Doctors are closely monitoring his progress because this is a first-ever treatment.
How do discoveries like this happen?
Breakthroughs like this do not occur overnight. They are the result of decades of research exploring biology, often without a clear or immediate medical application in mind.
The research on bacteria that led to the discovery of CRISPR probably sounded unimportant, perhaps even trivial, at the time, but it laid the foundation for a revolution in medicine.
In the US, most of this basic foundational research has been funded by the federal government via the National Institutes of Health (NIH) and the National Science Foundation (NSF). Stories like those shared here serve as a reminder that science investments can lead to discoveries that one day change lives. Unfortunately, the United States’ ability to remain a leader in this area is uncertain. The current administration is proposing changes to research guidelines and federal grant funding that could impair the ability to pursue fundamental questions that lead to new treatments and cures.
Who has access?
While CRISPR is promising, developing a single treatment using it can take more than a decade and cost billions of dollars. Many possible treatments also fail before ever getting final approval from the FDA.
For the therapies that do make it, the costs for patients can be high. This is especially true for rare diseases, which have fewer potential patients who may benefit.
For example, both Casgevy and Zolgensma are estimated to cost about $2 million per treatment. While these treatments are considered one-time-only, their price tags still raise difficult questions.
How can patients and families afford these treatments? Will insurance cover them? How can we ensure that these advances benefit everyone, not just those who can pay? Which treatments should be prioritized, and which should not?
We don’t have answers yet, but these are the types of questions society must grapple with as we move into this new era of medicine.





This is why federal funding is vital. Scientists and researchers need access to funding to try out different theories without the fear of failure. Many believe with federal funding cut that companies can step in, but most companies are more cautious about spending bc they want to make sure investments are worth it.
The first thing that came to mind after reading this article is that corporations have tremendous R&D (research and development) costs that need to be charged initially to keep their doors open and , although the cure may be administered to a patient with a rare disease , the corporation must be compensated accordingly for all the reasons aforementioned. That's the cold hard facts of the matter as I see it and this is where the government or philanthropy comes into play to cover these costs. To know that there is a cure but only for the super rich, is heartbreaking ,especially when a child of poor parents is involved, thus if someone can't afford to pay for the cure, the child dies of a disease that could have been cured of the ailment after the drug had been administered. In a single payer health care system , this would not be a problem as the government would pay for the procedure off of taxpayer's dollars after establishing a more equitable price for the treatment through negotiations between the producer and the government to the benefit of all concerned.
I would heartily approve of such a system in the USA.